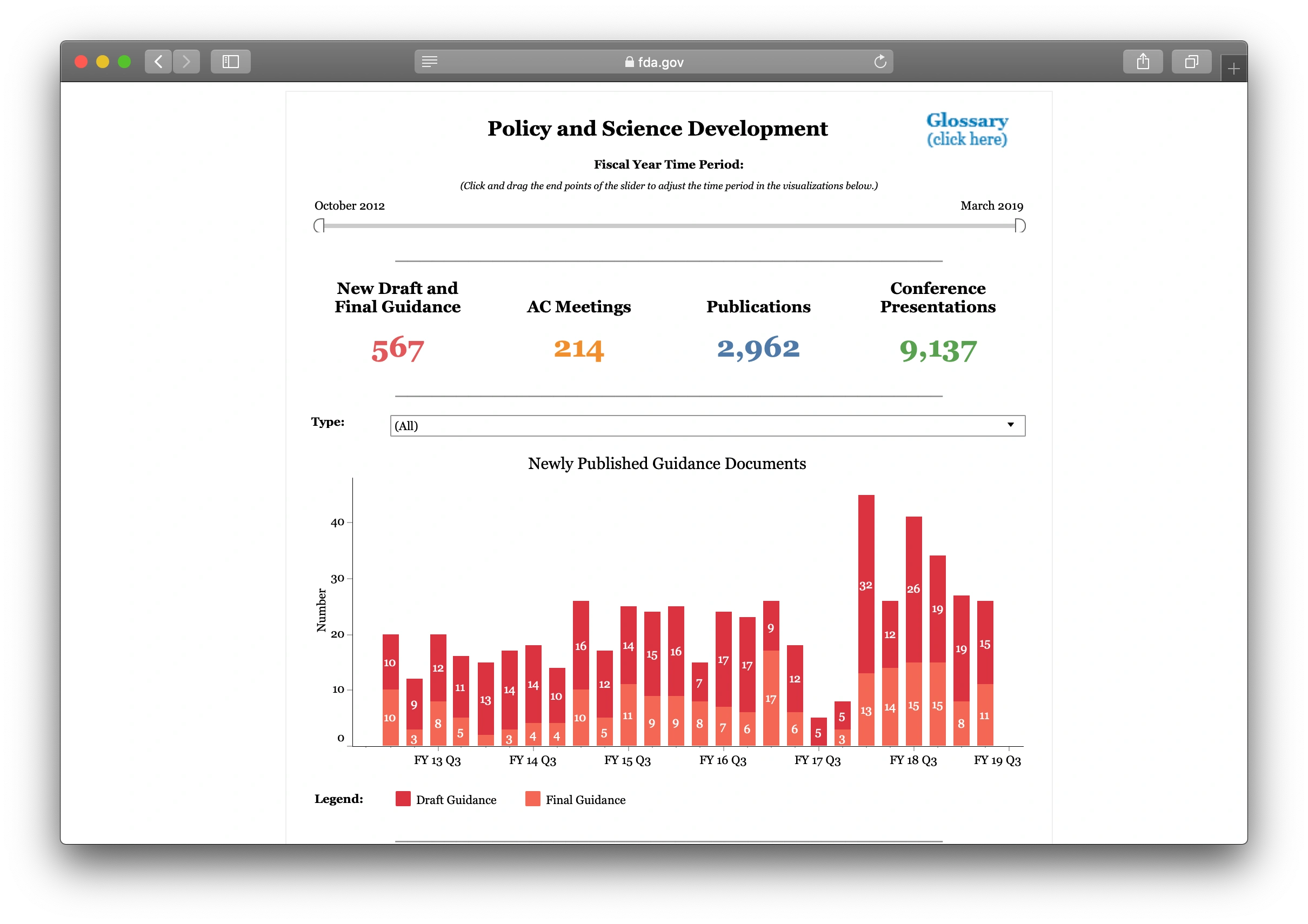
In late May CDER launched a new web page for their portion of FDA-TRACK, the performance management system used by the FDA to monitor the agencies’ programs. The new web page provides business intelligence and analytics for the division using 7 dashboards:
- Policy and Science Development
- Drugs and Biologics (Pre-Approval)
- Biosimilars (Pre-Approval)
- Generics (Pre-Approval)
- Drug Shortages (Post-Approval)
- Compliance (Post-Approval)
- Patient Safety Tools (Post-Approval)
Each dashboard shows graphics with clickable links to more detail – see the example from the Policy and Science Development dashboard showing meetings and publications above. Hovering over clickable items produces a popup that contains links to more detail, and additional links and information are included below the graphics. More details on the new pages are available in this newsletter. The new page and its dashboards should enable improved public transparency for CDER performance.
Text Copyright © 2019 Katrina Rogers
