What It Is and Why We Need It
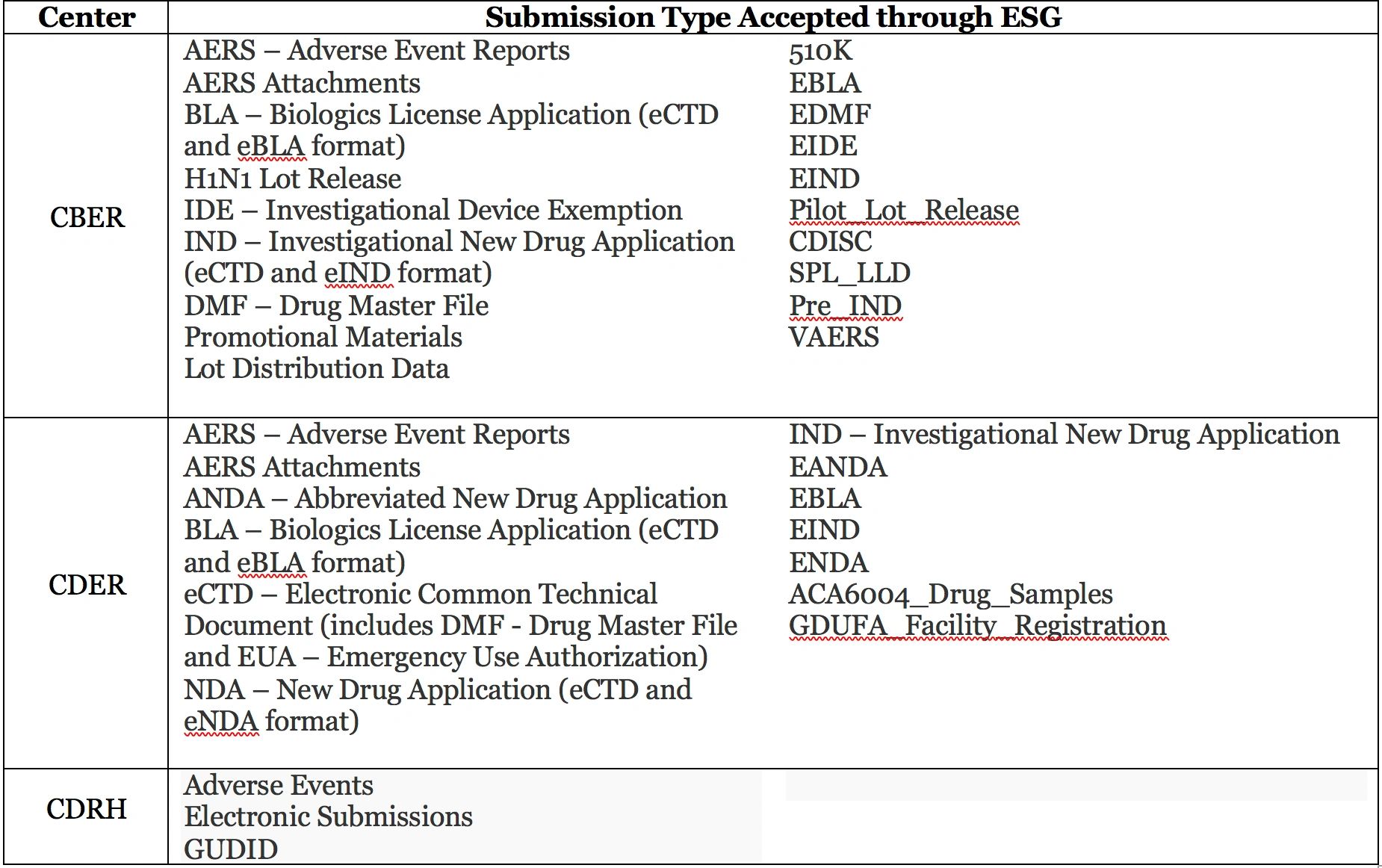
The FDA’s Electronic Submissions Gateway (ESG) is a tool that facilitates the secure transmission of electronic submissions from industry partners to the agency. The Gateway is not part of the actual review process; instead, it provides a means for receiving and acknowledging a submission, routing the submission file to the appropriate Center’s receiving system, and notifying Center personnel that the submission is available for review. There are two ways to use the ESG; a web portal designed for low-volume users (known as Web Trader) and a system-to-system connection (known as AS2) that can handle a higher volume of submissions but requires the installation of a software gateway at the user end. Both types require establishing user accounts, digital certificates, and digital signature documentation. Either system allows users to make submissions at any time of day (submissions made after FDA business hours are received and processed more quickly, according to ESG statistics). This table above shows the types of submissions that are accepted by CDER, CBER, and CDRH through the ESG.
The eSubmitter tool discussed in an earlier post may be used to package some of these submissions before secure transmission through the ESG. As the agency continues to move towards fully electronic submissions, we may expect modifications to the ESG to handle more submission types. It would also be helpful if the submission type tables for the different pieces of software were better linked to show specifically what tools are available for specific submissions.
Text and Image Copyright © 2018 Katrina Rogers
