The Common Technical Document Provides a Structure for Submission
(Note: This post is part of a multi-part series on the CTD this fall)
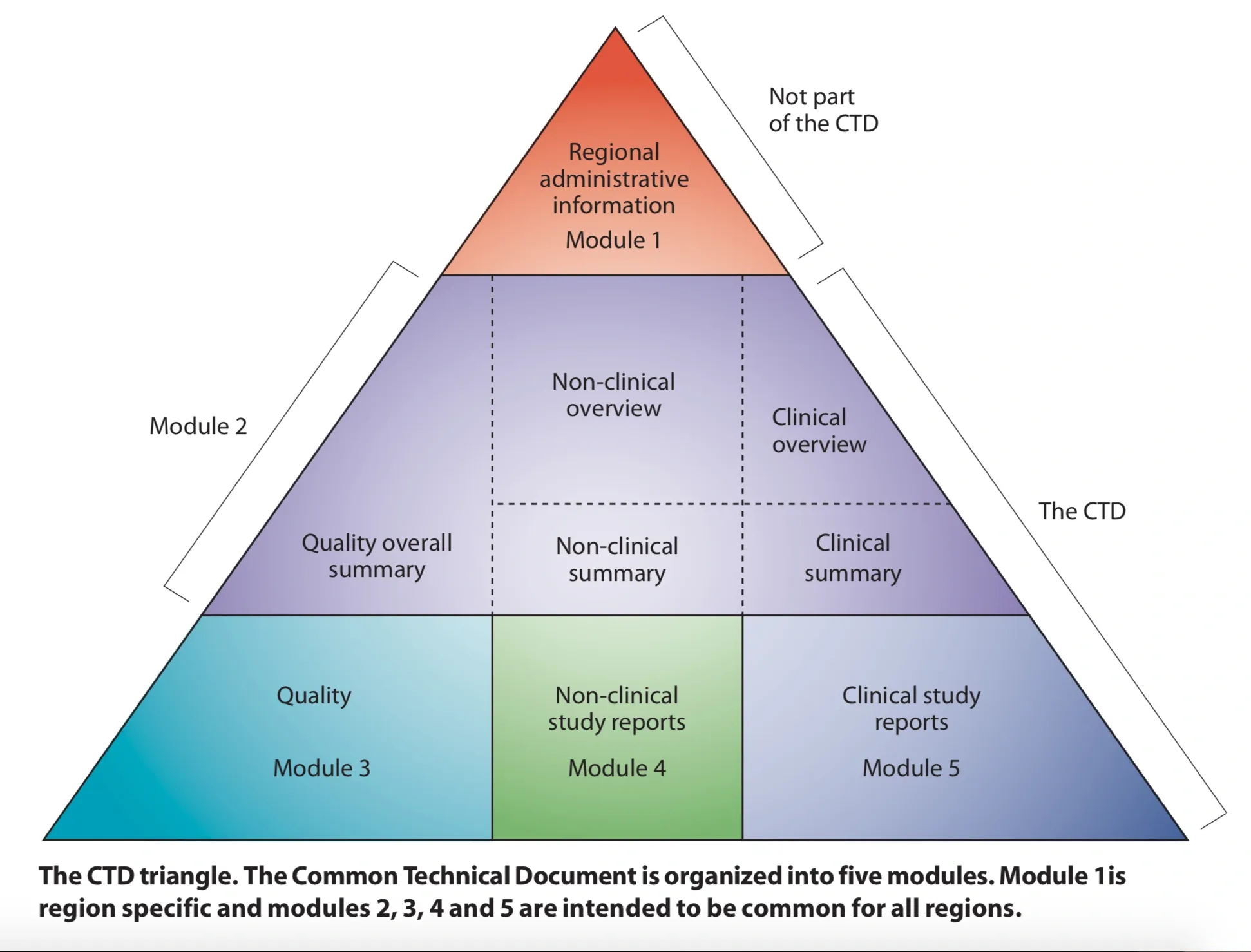
Before it became electronic, the common technical document (or CTD) was proposed by an ICH steering committee as a means to efficiently combine the efficacy, quality, and safety data for a drug application into a single format that can be used for harmonized regulatory submissions. The common format also improves the efficiency of regulatory review. There are 4 parts, or modules, to the CTD. The first module is for administrative and prescribing information the is specific to the ICH region for the submission. Module 2 presents the summary data, and modules 3-5 present the detailed quality data, nonclinical study reports, and clinical study reports, respectively. The graphic at the top of this post (from the ICH CTD page) shows the hierarchy of the modules. This format became the official format for drug and biologic submissions in the EU in 2003 and was strongly recommended in the US. We’ll discuss the history of the CTD and eCTD in my next post.
Reach out to me if you want to know more or discuss your medical product development challenges.
https://calendly.com/katrinarogers
Follow the links below to read each part of the series.
Electronic Regulatory Submissions Part 1
Electronic Regulatory Submissions Part 2
Electronic Regulatory Submissions Part 3
Electronic Regulatory Submissions Part 4
Electronic Regulatory Submissions Part 6
Electronic Regulatory Submissions Part 7
Electronic Regulatory Submissions Part 8
Electronic Regulatory Submissions Part 9
Electronic Regulatory Submissions Part 10
Electronic Regulatory Submissions Part 11
Electronic Regulatory Submissions Part 12
Electronic Regulatory Submissions Part 13
Electronic Regulatory Submissions Part 14
