CTD Organization Makes Finding Information Simpler
(Note: This post is part of a multi-part series on the CTD this fall)
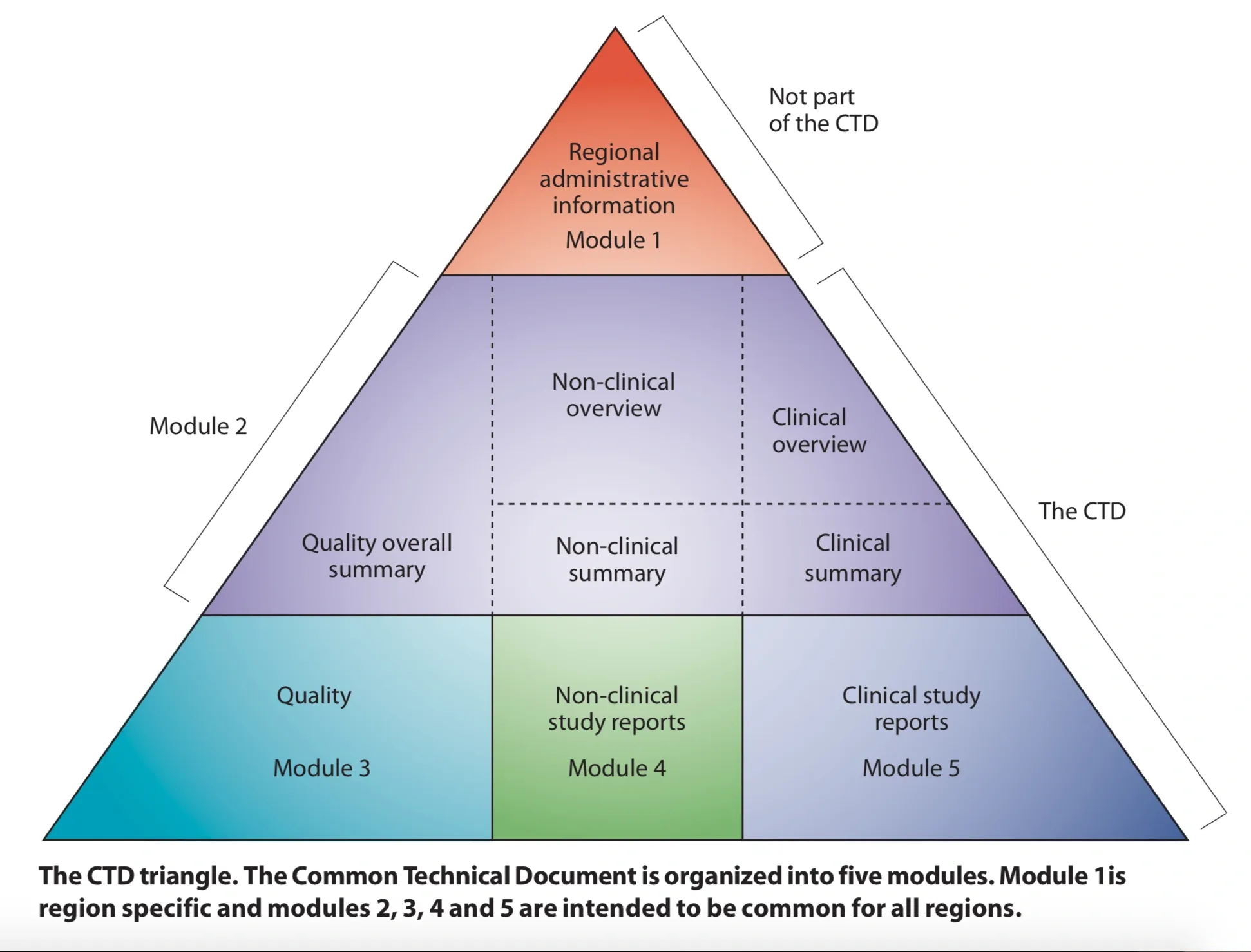
Part of what makes the CTD so useful is its internal structure. We can look at the Quality Overall Summary (QOS) as an example (see the M4Q guidance for reference). The guidance table of contents shows the exact structure of the QOS and each section or subsection of the actual application will be numbered as shown in the table of contents. For example, in the QOS, which will be section 2.3 (corresponding to Module 2 section 3), the drug substance is described in section 2.3.S, with subsections 2.3.S.1-7 giving the individual parts of the summary for drug substance. One can quickly recognize section P must represent drug product, and sections A and R quite naturally lend themselves to Appendices and Regional Information. This module.section.subsection format applies to the entire CTD, and enables rapid identification of any part of the document based on these identifiers. In my next post I’ll discuss what makes the eCTD work.
Reach out to me if you want to know more or discuss your medical product development challenges.
https://calendly.com/katrinarogers
Text Copyright © 2019 Katrina Rogers
Follow the links below to read each part of the series.
Electronic Regulatory Submissions Part 1
Electronic Regulatory Submissions Part 2
Electronic Regulatory Submissions Part 3
Electronic Regulatory Submissions Part 4
Electronic Regulatory Submissions Part 5
Electronic Regulatory Submissions Part 6
Electronic Regulatory Submissions Part 7
Electronic Regulatory Submissions Part 8
Electronic Regulatory Submissions Part 9
Electronic Regulatory Submissions Part 10
Electronic Regulatory Submissions Part 11
Electronic Regulatory Submissions Part 13
Electronic Regulatory Submissions Part 14
