Among the many resources made available by the FDA, the Orange, Purple, and Green Books stand out for their names and content. These books address public demand for lower-cost drug products, which prompted laws and regulations requiring lists of alternatives. In addition, each book covers its area according to the regulatory framework for its respective products. For example, the Orange Book covers chemical drug products, the Purple Book biologicals, and the Green Book veterinary medicines. This month’s blog post looks at the history and content of each of these color books.

The FDA publishes numerous resources for people wishing to create medicines and medical devices. One set of resources is for companies wanting to develop a generic version of an existing medication. The name of these resources is the color books, and there are three: Orange is for chemical drugs, Purple is for biologicals, and Green is for veterinary medicines. Each color book offers essential information on its category, yet they are not well known. In this article, I will review the purpose of each color book and how you can access them for your information or generic development plan.
The Orange Book is the Grandparent of the FDA color books, celebrating its 40th anniversary in 2020. The products listed in the Orange Book have an application with an approval that has not been withdrawn for safety or efficacy reasons. It also includes a list of therapeutic equivalents for prescription drugs. Almost all states have adopted legislation or regulations encouraging the substitution of drug products to control costs. In the late 1970s, individual states would submit requests to the FDA for assistance in creating their lists of substitute drug products, known as formularies. It didn’t take long for the FDA to realize that one list based on standard criteria would be a better way to serve the public need. Hence, the Orange Book was proposed and first published in October 1980. Subsequent amendments to the FD&C Act have added reporting requirements that are now part of the Orange Book. For example, the FDA included approved over-the-counter drugs and biologic drug products approved under section 505 in the Orange Book because of the Hatch-Waxman Amendments of 1984. The Orange Book also contains a list of approved products that have never been marketed, are for exportation or military use or have been discontinued from marketing without being withdrawn for safety or effectiveness reasons. (1)
The Orange Book also has an Addendum that includes patent and exclusivity information for the drug products listed in the primary Book. Thus, sponsors may use the Orange Book to track innovative drugs that may be good targets for generic drug development. The print edition is issued annually in January. Updates to the Orange Book are frequent, occurring daily, semimonthly, and monthly on the website. Updates for the data files and cumulative print supplements are released monthly. Newly approved NDA drug products will generally appear in the updates during the month following their approval. In addition, the FDA has published a Q&A guidance about the Orange Book and guidance on how the agency determines therapeutic equivalence. Fans of the Orange Book may want to view the Orange Book’s 40th Anniversary webinar, too.

The FDA licenses biologic drug products under the Public Health Service (PHS) Act. The PHS definition of a biological product is a “virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood component or derivative, allergenic product, or analogous product, … applicable to the prevention, treatment, or cure of a disease or condition of human beings.” However, they still meet the definition of a drug under the FD&C Act, so both the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER) have regulatory responsibility. However, the pathway for biological product approval is known as a Biologics License Application (BLA) per section 351 of the PHS Act. The reason for this difference is biologics are defined by their controlled manufacturing process rather than by the identity of the active component (which may be very difficult to determine). Once biological products became more important as treatment options, the US needed a way to approve generic biologics. Therefore, Congress passed the Biologics Price Competition and Innovation Act of 2009 (BPCI Act). “The BPCI Act created both the abbreviated licensure pathway for biologics allowing for the licensure of biosimilar and interchangeable biologics, and for the first time created an exclusivity period for certain qualifying innovator biological products.”(2) This act gave sponsors a way to file for approval of a biosimilar product (one highly similar to a reference biologic) or an interchangeable product (one that is both biosimilar and expected to produce the same clinical result as the reference biologic). Readers may find more information on biosimilars here and answers to some common questions about therapeutic biological products here.
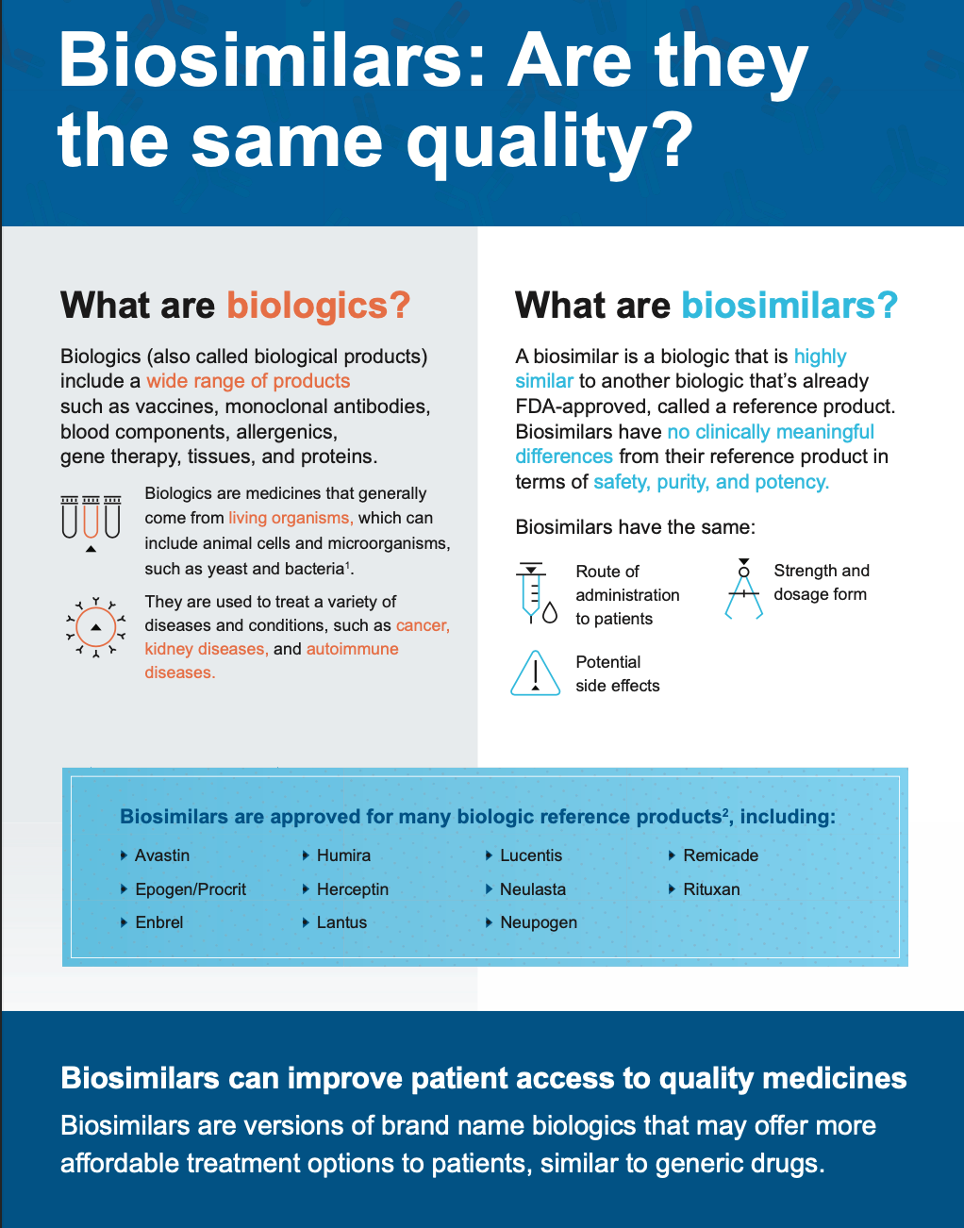
You won’t be surprised that the Purple Book lists both reference biologics side by side with all corresponding licensed biosimilar and interchangeable products. The name comes from a staff suggestion during a meeting on the book’s creation. Also included is information about the date of the first licensure and the expiration date of the exclusivity period for the reference product. However, you won’t find information about exclusivity for biologics designated as orphan products. Instead, search the Orphan Drug Designations and Approvals database for that information (see this website). The FDA is quick to point out that the Purple Book is not the biological equivalent to the Orange Book because the laws governing biologic licensure are different from those for drug approval. However, anyone may use the Purple Book to look up product label information for reference, biosimilar, and interchangeable products. Sometimes this information includes practical detail like the summary review and medication guide (see the example search below).

You may be surprised to learn that the FD&C Act also applies to drugs intended to treat animals (both companion and food-producing). Until the passage of the Generic Animal Drug and Patent Restoration Act (GADPTRA) in late 1988, there was no process for generic animal drug approval. GDAPTRA established the legal framework for Abbreviated New Animal Drug Applications (ANADAs) and directed the FDA to develop the regulations and resources for generic animal drug sponsors. The Green Book was first published in January 1989 to meet GADPTRA requirements. Like generic human medicines, a new generic animal drug must contain the same active ingredient in the same concentration, dosage form, and route of administration as the reference animal drug. The labeling must also be the same as the reference animal drug, with obvious exceptions like the tradename, logo, and sponsor. The Green Book provides information on the reference drugs, including their active ingredients and any exclusivity periods granted for new uses or claims. The Green Book does not include therapeutic equivalents for the reference animal drugs. However, all the existing approved products for a particular active compound will be listed, including their dose forms. Sponsors of proposed generic equivalents use Green Book information to develop their ANADAs (here’s an overview of the ANADA process). Readers may find more information about animal drugs and their approval process here.

All three color books are available online or in downloadable electronic versions. The Orange Book is the only one available in print format and even has a mobile app. You can find it by searching for Orange Book Express 2.0 in your phone’s app store. Readers may find it helpful to try a few searches to understand how the books work, so I’ve shared a few examples after each Book’s link below.

- Orange Book – try searching for “Kyprolis,” an anti-cancer medication used to treat multiple myeloma. Onyx Therapeutics, currently a subsidiary of Amgen, developed this medication. Unfortunately for cancer patients, Kyprolis is still under patent exclusivity, with the earliest expiration in 2025. That makes it a potential target for a generic version.
- Purple Book – try a simple search for “Epogen,” Amgen’s biological product used to treat anemia in chemotherapy patients. You’ll find it has one biosimilar and no interchangeable products.
- Green Book – Navigate to Animal Drugs @ FDA and try searching for “spectinomycin.” Spectinomycin is an older antibiotic with a wide range of activity with several approved dose forms, primarily for food-producing animals. Bimeda Animal Health received approval for ANADA 200-694 in September of this year.
The three FDA color books provide the information companies need to create generic versions of approved drug products. They are also helpful for pharmacists, healthcare practitioners, and members of the public interested in looking for generic alternatives for brand-name medications. The processes for generic approval depend on the laws and regulations governing chemical, biological, or animal drugs. Nevertheless, they are similar enough to facilitate adapting the original publication (the Orange Book) to meet new requirements (as the Green and Purple Books). The future may hold additional color books as therapeutic modalities continue to develop.

(1) USFDA (2022). The Orange Book Preface, (January 19, 2022). Retrieved from https://www.fda.gov/drugs/development-approval-process-drugs/orange-book-preface
(2) Renu Lal, Pharm. D. (2014). The Purple Book [Electronic newsletter]. FDA/CDER SBIA Chronicles, (November 18, 2014), 2. Retrieved from https://www.fda.gov/media/90150/download
If you enjoyed this article and would like to read more by Katrina, sign up for her newsletter.
