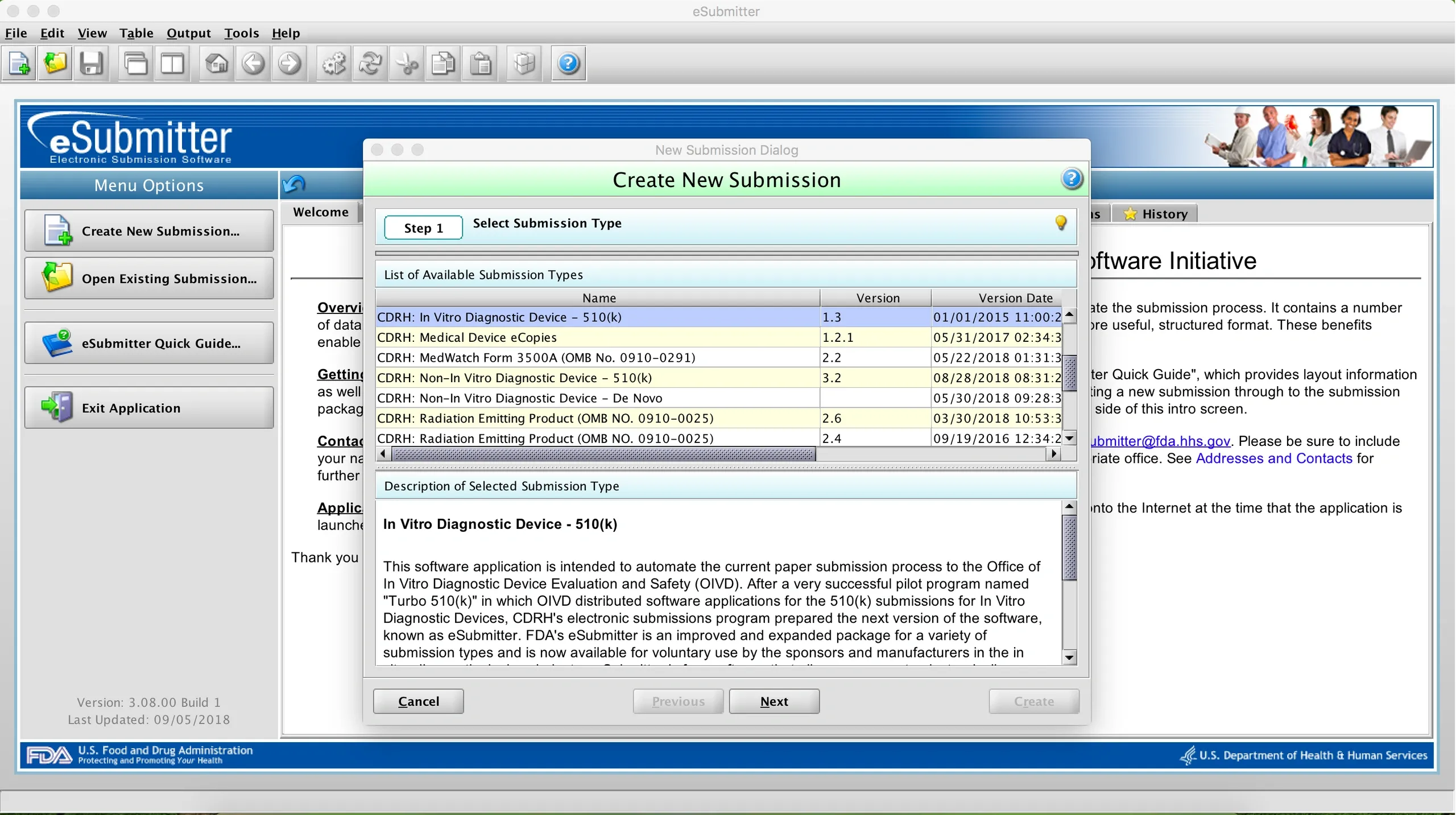
In December, the FDA issued a final rule revising all the regulations for medical device regulatory submissions to replace paper with electronic copies. An update to the eCopy Guidance was issued with the rule, along with updates to guidance on Premarket Approval Applications and Supplements to align them with the eCopy requirements (see the full list here — look for guidance issued on December 16, 2019). Sponsors may use any method they choose to produce an eCopy that meets the technical standards in the eCopy Guidance. You may want to consider using the free eCopies-eSubmitter tool available from the FDA. Read more about it in this previous post.
Reach out to me if you want to know more or discuss your medical product development challenges.
https://www.linkedin.com/company/katrina-rogers-consulting-llc
https://calendly.com/katrinarogers
Text and Image Copyright © 2020 Katrina Rogers
