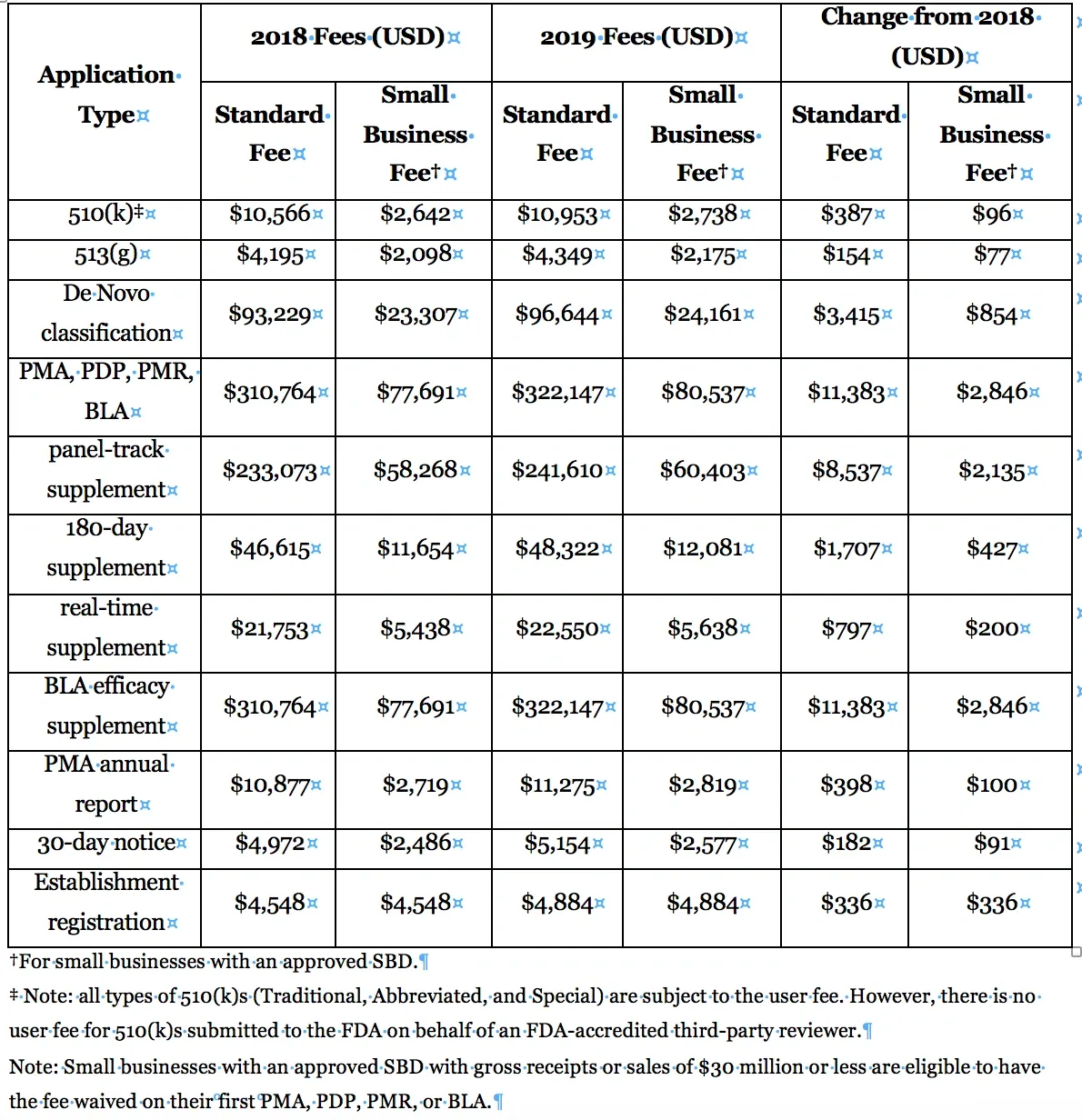
The FDA’s CDRH branch posted the 2019 rates and payment procedures for Medical Device User Fees on July 30, 2018. See the table below for a comparison of the 2018 and 2019 fees. Most of these fees are set using a formula that references the standard fee for a premarket application, which will increase to $322,147 USD in 2019. The rates are heavily discounted for businesses (those with gross sales or receipts no more than $100M USD); this discount makes the annual qualification process for this classification worthwhile. The annual fee for establishment registration is $4,884, and all establishments must pay this fee regardless of their size. The fiscal year 2019 runs from October 1, 2018 – September 30, 2019. Here’s a table of last year’s fees compared to this years:
Read the full notice on 2019 Medical Device User Fees, including how they were calculated, payment procedures, and qualifying for reduced small business fees, here.
Text and Image Copyright © 2018 Katrina Rogers
