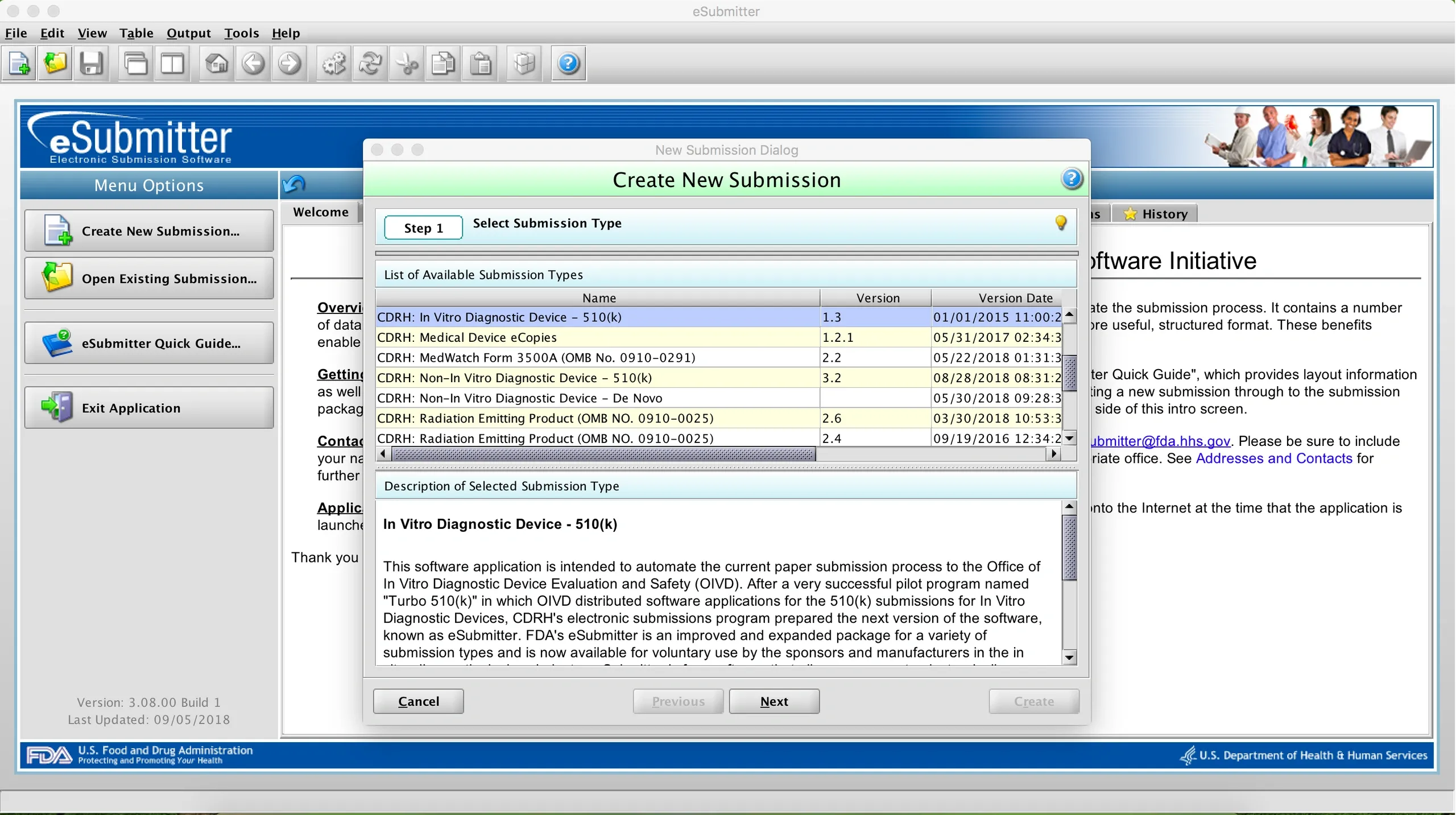
The FDA offers a free software package called eSubmitter designed to help companies prepare electronic documents for selected regulated submissions (for example, CBER Annual Reports or CDRH eMDR reporting under form 3500A). The tool creates a package of documents on your local computer that may be uploaded to the appropriate Center’s office using a secure mode (for example, through the Electronic Submissions Gateway). eSubmitter is a voluntary tool, and it does not need further user validation when used for the submission types listed on the eSubmitter table. This list of approved submission types are also listed in the dialog for the New Submission workflow; if it’s not in the list, the tool will not provide a validated output for your submission.
Once a submission type is selected, the tool guides you through a checklist of questions and items required for that particular submission. Features of the tool include:
- Fields that let you look up the appropriate answer from a submission specific list of answers (for example, device product codes or guidance referenced in the submission)
- Support for the MedDRA dictionary (you will need a subscription to the dictionary to use this feature)
- Address and Contacts lists where you may save information on your facilities and personnel for use in multiple submissions
- An area to maintain a set of Master Files for your submissions
- Fields to associate FDA-assigned ID numbers with your submission documents
- The ability to save a draft submission for update at a later time
The tool tracks the items you’ve completed for each submission and reports can be run to identify missing items. Once all the required items are complete, you can package the files, which creates the complete submission file (in .zip format) and a cover letter. Finally, the tool will provide instructions on how to transmit the packaged submission to the appropriate center.
The software runs on Windows and Mac systems, though you’ll need to follow the special instructions for installation on a Mac (the installation went well on my MacBook Pro). You will also need a PDF reader and browser, as well as 30 MB of space for installation. Also helpful is an ability to create PDF files to upload into the tool for inclusion in your submissions, and there are specific version requirements for these files (check your documentation).
If you use the tool to create and store electronic records for purposes other than those on the table, you may be responsible for other regulatory requirements including validation, change control, and electronic signatures. The software documentation clarifies that the agency intends to provide additional submission types as part of future updates, so be sure to check that you have the latest version before you start work on your submission. You can find the latest version of the tool here.
Text and Image Copyright © 2018 Katrina Rogers
