Chapter 6 – Biomedical Mobilization Against COVID-19
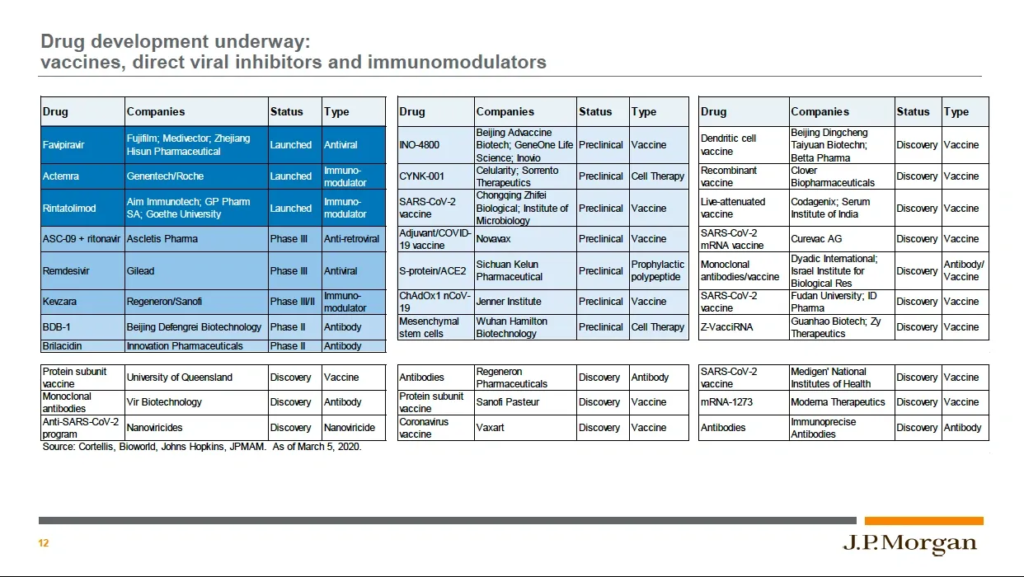
The response of our biomedical community to the novel coronavirus outbreak has been impressive, and is illustrated in this slide from a JP Morgan webcast on COVID-19 and the Markets held on March 20th (see here for more information on the subject from some very smart people at JP Morgan). The 64 treatments or vaccines in this table were in the various stages of development shown as of March 5th and represent a broad engagement against a number of targets. Readers already know that development can be a long game, taking 13 years or longer from discovery to approval, but several of the potential treatments (like chloroquine) are already approved for other indications, and remdesivir, the medicine currently supported by the CDC and WHO as the best potential treatment, is in phase 3 clinical trials right now. WHO has also launched a massive multi-country clinical trial called SOLIDARITY with the goal of comparing multiple potential treatments to each other. It’s up to us to do our part by following the requirements of our public health agencies and Doing the Five to protect ourselves, our families, and our communities.
Reach out to me if you want to know more or discuss your medical product development challenges.
Text Copyright © 2020 Katrina Rogers
Share this post:
