Pharmaceutical manufacturing has traditionally been a batch-based process, with different stages of production being conducted in discrete steps. However, in recent years there has been an increasing interest in continuous manufacturing as an alternative to batch-based production. Pharmaceutical advanced manufacturing also includes distributed and point of care modes as well as using tools like artificial intelligence and advanced networking. In this post we’ll look at the regulatory and economic considerations that need to be taken into account when considering a switch from batch to continuous production.
We hear a lot about advanced manufacturing (I’ll abbreviate it as AM) in the press. This term covers a lot of ground because it includes “production activities that depend on information, automation, computation, software, sensing, and networking.” (1) One or more of these concepts apply to modern pharmaceutical production, so how is AM different for drugs and biologics? We’ll review continuous, distributed, and point-of-care manufacturing as examples where AM techniques and tools will change how we think about making pharmaceuticals. Note: Some of what I’ll discuss in this article can apply to both drug substance and drug product manufacturing, and some to one or another step.
Pharmaceutical manufacturing has traditionally been a batch-based process, with different stages of production (formulation, blending, compression, coating, packaging, etc.) being conducted in discrete steps. However, in recent years there has been an increasing interest in continuous manufacturing as an alternative to batch-based production. Continuous manufacturing (CM) offers several potential benefits over batch production, including improved process control, reduced lead times, and increased flexibility. Additionally, it could potentially reduce costs, as the physical equipment and space used in production are projected to be much lower than in batch-based processes. CM has been adopted in many industries, but the pharmaceutical sector has been a slow adopter. One of the reasons frequently cited as a barrier to adoption of CM and other AM technologies is the perception that the regulatory outcomes will be poor. The FDA, in tandem with international regulatory bodies, has created several resources to address this perception.
- The draft guidance “Quality Considerations for Continuous Manufacturing,” released in 2019, provides the agency’s “current thinking on quality considerations for continuous manufacturing of small molecule, solid oral drug products. . . .”
- The draft guidance “Q13 Continuous Manufacturing of Drug Substances and Drug Products,” released in 2021, is the FDA’s publication of the ICH Step 2 guidelines for public comment. The ICH document, which reached Step 4 last November, clarifies CM concepts, describes scientific approaches, and presents regulatory approaches to implementation. ICH Q13 is being implemented in Europe with a target date of July 10, 2023.
- ICH has published a concept paper as the first step toward a training program to facilitate implementation of CM processes.
- CDER’s Emerging Technology Program, which allows industry representatives to meet with members of the FDA quality review and inspection programs to “discuss, identify, and resolve potential technical and regulatory issues regarding the development and implementation of a novel technology prior to filing a regulatory submission.” This innovative program has a straightforward application process and participants can discuss technologies for which the FDA has limited review or inspection experience. See Figure 1 for the agency’s view of the lifecycle of an emerging technology within the program.
- CDER’s FRAME Initiative, which is assessing existing guidance, regulations, and statutory authorities for areas of policy consideration. The FRAME initiative is prioritizing four technologies from the National Academies of Sciences, Engineering, and Medicine (NASEM) 2021 report on pharmaceutical manufacturing innovations. (2) These are:
- “End-to-End Continuous Manufacturing (E2E CM): fully integrated process into which raw materials are continuously fed and transformed and finished drug products are continuously removed.
- Distributed Manufacturing (DM): A decentralized manufacturing platform with manufacturing units that can be deployed to multiple locations.
- Point-of-Care Manufacturing (POC): A DM platform with manufacturing units in proximity to patient care; for example, at healthcare facilities.
- Artificial Intelligence (AI): Software and hardware systems that can perceive the environment, interpret data, and decide actions.” (3)
The NASEM report is worth reading – there are multiple problem statements worthy of research and entrepreneurism in the document, along with recommendations for CDER to facilitate adoption of novel manufacturing approaches. FRAME is the way CDER is implementing these recommendations. The first activity was the publication of a discussion paper on DM and POC manufacturing in October 2022, followed by an FDA/PQRI workshop on the regulation of these innovations.
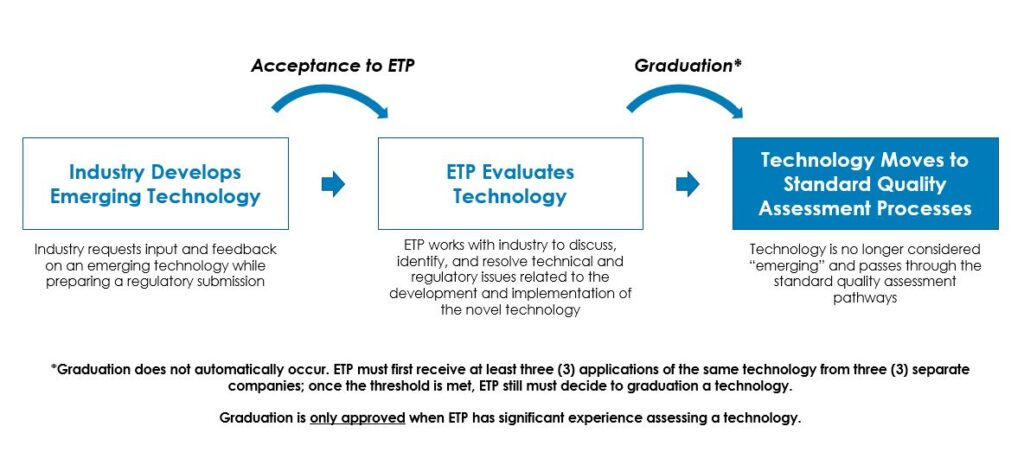
Of these resources, I think the Emerging Technology Program offers the best return for sponsors and manufacturers considering manufacturing innovations for their products. Naturally, you will want to read the guidance documents first to formulate your questions.
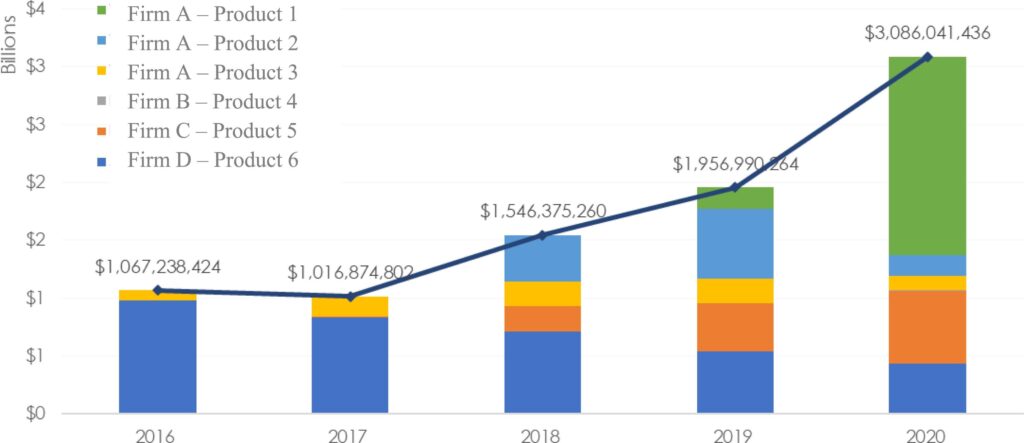
How has this regulatory guidance been implemented to date? A team led by OPQ Director Adam Fisher performed a self-audit of CM regulatory submissions from 2015 to 2022. Their definition of CM was an “integrated process consisting of a series of two or more unit operations,” and six applications fit this profile. The published article is full of valuable details like the first figure, which illustrates the annual sales of these six products (reproduced here as Figure 2). The authors concluded there were “no substantial barriers associated with common regulatory interactions (e.g., time to approval, postapproval change reporting, inspectional scrutiny) related to implementation of CM as compared to batch manufacturing.” They also noted “. . . engagement with the Emerging Technology Program has been a clear advantage for the adoption and implementation of CM.” While four of the six applications were granted Breakthrough Therapy designation (with expedited review times), the authors observe this demonstrates the agency is capable of approving CM products within the expedited review times of this program. These products demonstrated the economic benefits of CM, because they “tended to reach the market sooner after regulatory filing, translating to earlier patient access to medicines and a potential $171–537 M in early revenue benefit.”
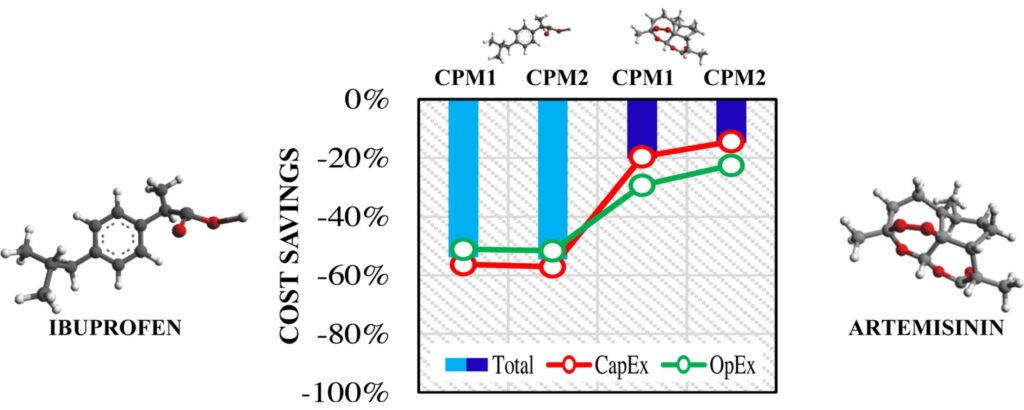
The Fisher et al paper includes a brief review of the CM implementation literature on economic factors. Two of these papers deserve further consideration here. A team led by Spencer Schaber evaluated the economics of a continuous process for API plus tablet drug product being developed by MIT researchers against batch production in a dedicated facility. (4) Their estimated savings were 20-76% for capital costs and 40% lower to 9% higher for operating costs. The process was still under development at press; therefore, several factors were estimated and process yields were unknown. Nevertheless, this study is frequently cited as an example of the economics of CM. The second paper presents an initial assessment of new CM processes for two recognized active ingredients, ibuprofen and artemisinin. (5) The purpose was demonstrating a method for evaluating CM potential and applicability. Their model found projected capital savings of up to 57% and 20% for the respective APIs, and operating savings of up to 52% and 29% (see Figure 3 from their abstract). They also found the waste produced per unit of product was decreased using the CM process. While both studies use processes that are approaching an implementation phase, neither is a start to finish case study for an approved product. The CDER team that produced the self-audit would do us all a favor if they organized an industry benchmarking study to report the economics of the six approved products using one or both of these methods.
Advanced manufacturing is more likely to affect the production of new drugs in the next few years. However, targeted research on CM-adaptable synthetic and biologic processes could drive progress in AM adoption for established therapeutics. We don’t have the 2023 CDER guidance agenda at the time of publication, so it’s not clear what new resources will be available from the FDA this year. What is clear is that pharmaceutical advanced manufacturing innovation requires a multidisciplinary team, any part of which may stimulate progress in aligned domains. The partnership of business functions like finance, sourcing, and talent cultivation and management is also critical to success. I challenge you to learn more about AM in drug and biologic development and engage with your regional partners to convert the innovations to practice.

(1) The definition of Advanced Manufacturing from Manufacturing.gov, accessed 27JAN2023. https://www.manufacturing.gov/glossary/advanced-manufacturing
(2) National Academies of Sciences, E., & Medicine. (2021). Innovations in Pharmaceutical Manufacturing on the Horizon: Technical Challenges, Regulatory Issues, and Recommendations. Washington, DC: The National Academies Press. Accessed 03FEB2023. https://nap.nationalacademies.org/catalog/26009/innovations-in-pharmaceutical-manufacturing-on-the-horizon-technical-challenges-regulatory
(3) FRAME Initiative website, accessed 03FEB2023. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/cders-framework-regulatory-advanced-manufacturing-evaluation-frame-initiative
(4) Schaber, S. D., Gerogiorgis, D. I., Ramachandran, R., Evans, J. M. B., Barton, P. I., & Trout, B. L. (2011). Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case Study. Industrial & Engineering Chemistry Research, 50(17), 10083-10092. Accessed 03FEB2023, https://pubs.acs.org/doi/10.1021/ie2006752
(5) Jolliffe, H. G., & Gerogiorgis, D. I. (2016). Plantwide design and economic evaluation of two Continuous Pharmaceutical Manufacturing (CPM) cases: Ibuprofen and artemisinin. Computers & Chemical Engineering, 91, 269-288. Accessed 03FEB2023, https://www.sciencedirect.com/science/article/abs/pii/S0098135416301016
If you enjoyed this article and would like to read more by Katrina, sign up for her newsletter
