February is Rare Disease Month because the 29th is the rarest of days. This month prompts a crucial exploration into the challenges of developing therapies for rare diseases. These conditions, often affecting children and encompassing serious or life-threatening disorders, pose significant unmet medical needs. This article aims to shed light on the regulatory landscape guiding the development of treatments for rare diseases, emphasizing the role of the U.S. FDA and global agencies. Whether you’re a researcher, healthcare provider, family member, or advocate, understanding these regulatory perspectives is critical to supporting the community and advancing patient-focused drug development.
Rare Disease Guidance
Most of the guidance documents for rare diseases come from the U.S. FDA. The overarching reference is “Rare Diseases: Considerations for the Development of Drugs and Biological Products, Guidance for Industry,” [1] which was issued in its final version in December 2023. This essential reference contains the agency’s current thinking on preclinical, clinical, and manufacturing considerations, lists relevant FDA engagement programs and tools, and makes summary recommendations for every step of a development program. It’s useful when creating the initial roadmap for your product, whether it’s a small molecule or biologic.

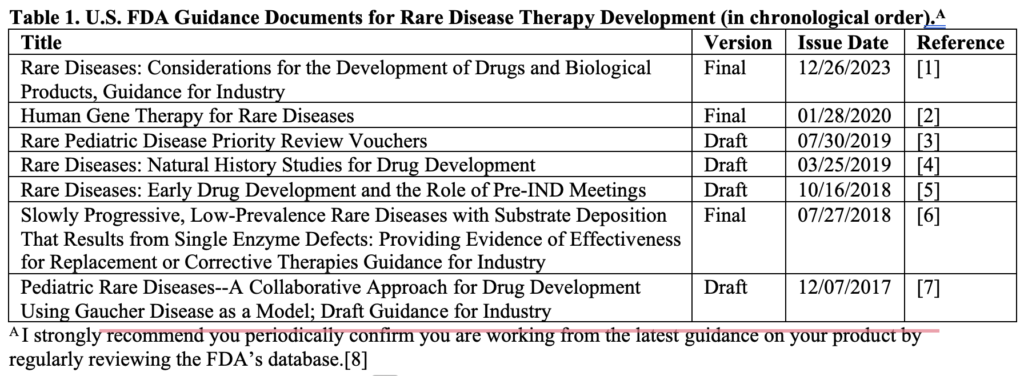
Six additional guidance documents are specific to rare disease therapy development (Table 1). All published guidance, whether draft or final, is applicable to development programs. The difference is whether the agency experts responsible for the guidance feel the regulatory science is sufficiently developed to issue a final version. Ignore a published draft guidance at your peril. I recommend review of all to assess and document their relevance to your therapy. Remember that good recordkeeping is essential to demonstrate to regulators when and why you made decisions during development.
Cellular and Gene Therapies
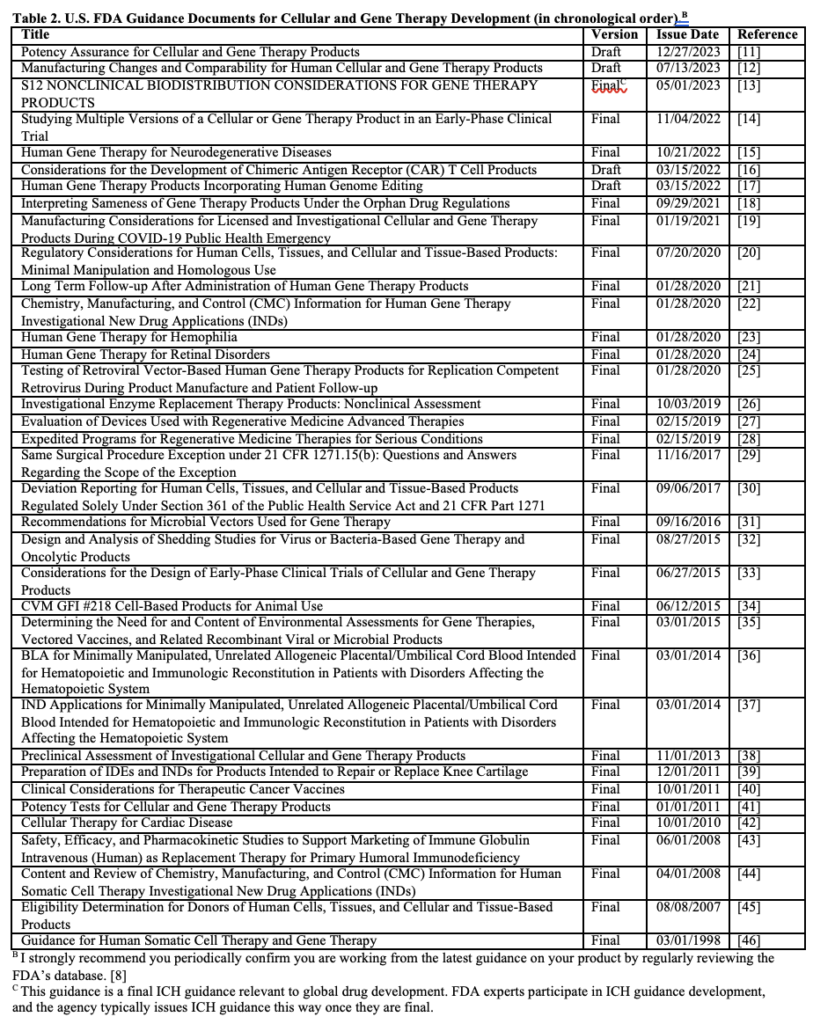
Reference 2 in Table 1 has a unique significance because cellular and gene therapies are the basis for numerous proposed rare disease therapies. Like reference 1, it is a summary guidance covering the agency’s thinking specific to preclinical, clinical, and manufacturing considerations for human gene therapies for rare diseases. It is the first guidance you should review when developing your cellular or gene therapy strategy. There are 36 additional documents relevant to this work as of January 15, 2024 (Table 2). Browsing this list tells us some interesting things. First, guidance was relatively slow to issue until January 28, 2020, clearly a red-letter day. The list tells us how regulatory science concerns developed and the focus areas of early cell/gene therapy research. A draft guidance for potency assurance will replace the existing final guidance issued in 2011. There’s even guidance for developing cell and gene therapies in animals, a market pivot I sometimes see, especially for companion animals.

The documentation for the 34 products on the FDA’s Approved Cellular and Gene Therapy Products list makes for fascinating reading and may be crucial to your strategy if your product is similar. [9] It’s also clear why certain guidance has been issued. For example, eight of the 34 approved products are based on cord blood, and two final guidance are in the table. There are many challenges remaining to be addressed, such as scaling up manufacturing for viral-vector-based therapies [10]; therefore, keep an eye out for additional documents.
Other FDA Resources
The FDA supports multiple programs that help rare disease therapy researchers. There are four approval approaches to making new drugs for serious diseases rapidly available. [47] These are:
- Fast Track – “a process designed to facilitate the development, and expedite the review of drugs to treat serious conditions and fill an unmet medical need.”
- Breakthrough Therapy – “a process designed to expedite the development and review of drugs which may demonstrate substantial improvement over available therapy.”
- Accelerated Approval – regulations that allow “drugs for serious conditions that filled an unmet medical need to be approved based on a surrogate endpoint.”
- Priority Review – a designation meaning the FDA has set a goal “to take action on an application within 6 months.”

In 2019, the FDA established the Rare Disease Cures Accelerator to support innovation and quality in the pipeline for rare diseases. [48] The purpose of this accelerator is to cooperate on pre-competitive projects, two of which have been launched:
- The Rare Disease Cures Accelerator – Data Analytics Platform (RDCA-DAP) supports rare disease characterization by allowing disease researchers and drug developers to access and analyze rare disease patient data. The Critical Path Institute has a global mandate and is leading this project (see below).
- Making four awards for rare disease assessment via the COA grant program, a pilot program to develop standardized clinical outcome assessments (COAs) as part of the FDA’s patient-focused drug development work.
Rare Disease Is Global
Across the world, regulatory agencies support research and development of treatments for rare diseases. The two biggest markets for medicines outside of the US provide excellent examples. The European Medicines Agency (EMA) has an orphan designation program providing incentives for drug innovators [49] and has issued guidelines for cell and gene therapy development [50]. The Japan Agency for Medical Research and Development (AMED) supports an Initiative on Rare and Undiagnosed Disease (IRUD) [51] and has a separate department for cell and gene therapies [52]. Nongovernmental organizations like the Critical Path Initiative collaborate globally on pre-competitive projects to advance medical, laboratory, and regulatory science to move medical discoveries more efficiently from the laboratory to consumers. [53] They have tools and resources beyond rare disease research and are worth monitoring for future developments. Orphanet is a consortium of 40 global countries providing high quality data related to rare diseases, clinical practice guidelines, and other resources connecting researchers to the patient community [54].

I hope this article has helped you better understand the regulatory perspective for drug development in rare diseases for whatever role you support the community (researcher, health care provider, family member, or advocate). We shouldn’t overlook the many organizations founded by patients and families who have transformed the research landscape and spearheaded the concept of patient-focused drug development. An excellent resource to explore them may be found on the National Organization for Rare Disorders website [55]. Lastly, I urge you to join a Rare Disease Day event this month and support this meaningful work.
As always, you can contact me to ask questions or dig deeper into any topic!
References
[1] U.S. FDA, “Rare Diseases: Considerations for the Development of Drugs and Biological Products, Guidance for Industry.” December 2023. Accessed January 12, 2024. https://www.fda.gov/media/119757/download
[2] U.S. FDA, “Human Gene Therapy for Rare Diseases.” January 2020. Accessed January 12, 2024. https://www.fda.gov/media/113807/download
[3] U.S. FDA, Rare Pediatric Disease Priority Review Vouchers.” July 2019. Accessed January 12, 2024. https://www.fda.gov/media/90014/download
[4] U.S. FDA, “Rare Diseases: Natural History Studies for Drug Development.” March 2019. Accessed January 12, 2024. https://www.fda.gov/media/122425/download
[5] U.S. FDA, “Rare Diseases: Early Drug Development and the Role of Pre-IND Meetings.” October 2018. Accessed January 12, 2024. https://www.fda.gov/media/117322/download
[6] U.S. FDA, “Slowly Progressive, Low-Prevalence Rare Diseases with Substrate Deposition That Results from Single Enzyme Defects: Providing Evidence of Effectiveness for Replacement or Corrective Therapies Guidance for Industry.” July 2018. Accessed January 12, 2024. https://www.fda.gov/media/136058/download
[7] U.S. FDA, “Pediatric Rare Diseases–A Collaborative Approach for Drug Development Using Gaucher Disease as a Model; Draft Guidance for Industry.” December 2017. Accessed January 12, 2024. https://www.fda.gov/media/109465/download
[8] U.S. FDA, Search for FDA Guidance Documents website. Accessed January 15, 2024 https://www.fda.gov/regulatory-information/search-fda-guidance-documents#guidancesearch
[9] U.S. FDA, Approved Cellular and Gene Therapy Products. Accessed January 12, 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
[10] Emily Capra, Andrea Gennari, Alberto Loche, and Carolin Temps, “Viral-vector therapies at scale: Today’s challenges and future opportunities.” March 29, 2022. McKinsey & Company. Accessed January 12, 2024. https://www.mckinsey.com/industries/life-sciences/our-insights/viral-vector-therapies-at-scale-todays-challenges-and-future-opportunities
[11]U.S. FDA, “Potency Assurance for Cellular and Gene Therapy Products.” December 2023. Accessed January 15, 2024. https://www.fda.gov/media/175132/download
[12] U.S. FDA, “Manufacturing Changes and Comparability for Human Cellular and Gene Therapy Products.” July 2023. Accessed January 12, 2024. https://www.fda.gov/media/170198/download
[13] U.S. FDA, “S12 NONCLINICAL BIODISTRIBUTION CONSIDERATIONS FOR GENE THERAPY PRODUCTS.” May 2023. Accessed January 12, 2024.
[14] U.S. FDA, “Studying Multiple Versions of a Cellular or Gene Therapy Product in an Early-Phase Clinical Trial.” November 2022. Accessed January 12, 2024. https://www.fda.gov/media/152536/download
[15] U.S. FDA, “Human Gene Therapy for Neurodegenerative Diseases.” October 2022. Accessed January 12, 2024. https://www.fda.gov/media/144886/download
[16] U.S. FDA, “Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products.” March 2022. Accessed January 12, 2024. https://www.fda.gov/media/156896/download
[17] U.S. FDA, “Human Gene Therapy Products Incorporating Human Genome Editing.” March 2022. Accessed January 12, 2024. https://www.fda.gov/media/156894/download
[18] U.S. FDA, “Interpreting Sameness of Gene Therapy Products Under the Orphan Drug Regulations.” September 2021. Accessed January 12, 2024. https://www.fda.gov/media/134731/download
[19] U.S. FDA, “Manufacturing Considerations for Licensed and Investigational Cellular and Gene Therapy Products During COVID-19 Public Health Emergency.” January 2021. Accessed January 12, 2024. https://www.fda.gov/media/145301/download
[20] U.S. FDA, “Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use.” July 2020. Accessed January 12, 2024. https://www.fda.gov/media/109176/download
[21] U.S. FDA, “Long Term Follow-up After Administration of Human Gene Therapy Products.” January 2020. Accessed January 12, 2024. https://www.fda.gov/media/113768/download
[22] U.S. FDA, “Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs).” January 2020. Accessed January 12, 2024. https://www.fda.gov/media/113760/download
[23] U.S. FDA, “Human Gene Therapy for Hemophilia.” January 2020. Accessed January 12, 2024. https://www.fda.gov/media/113799/download
[24] U.S. FDA, “Human Gene Therapy for Retinal Disorders.” January 2020. Accessed January 12, 2024. https://www.fda.gov/media/124641/download
[25] U.S. FDA, “Testing of Retroviral Vector-Based Human Gene Therapy Products for Replication Competent Retrovirus During Product Manufacture and Patient Follow-up.” January 2020. Accessed January 12, 2024. https://www.fda.gov/media/113790/download
[26] U.S. FDA, “Investigational Enzyme Replacement Therapy Products: Nonclinical Assessment.” October 2019. Accessed January 15, 2024. https://www.fda.gov/media/131295/download
[27] U.S. FDA, “Evaluation of Devices Used with Regenerative Medicine Advanced Therapies.” February 2019. Accessed January 15, 2024. https://www.fda.gov/media/120266/download
[28] U.S. FDA, “Expedited Programs for Regenerative Medicine Therapies for Serious Conditions.” February 2019. Accessed January 15, 2024. https://www.fda.gov/media/120267/download
[29] U.S. FDA, “Same Surgical Procedure Exception under 21 CFR 1271.15(b): Questions and Answers Regarding the Scope of the Exception.” November 2017. Accessed January 15, 2024. https://www.fda.gov/media/89920/download
[30] U.S. FDA, “Deviation Reporting for Human Cells, Tissues, and Cellular and Tissue-Based Products Regulated Solely Under Section 361 of the Public Health Service Act and 21 CFR Part 1271.” September 2017. Accessed January 15, 2024. https://www.fda.gov/media/107703/download
[31] U.S. FDA, “Recommendations for Microbial Vectors Used for Gene Therapy.” September 2016. Accessed January 15, 2024. https://www.fda.gov/media/94200/download
[32] U.S. FDA, “Design and Analysis of Shedding Studies for Virus or Bacteria-Based Gene Therapy and Oncolytic Products.” August 2015. Accessed January 15, 2024. https://www.fda.gov/media/89036/download
[33] U.S. FDA, “Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products.” June 2015. Accessed January 15, 2024. https://www.fda.gov/media/106369/download
[34] U.S. FDA, “CVM GFI #218 Cell-Based Products for Animal Use.” June 2015. Accessed January 15, 2024. https://www.fda.gov/media/88925/download
[35] U.S. FDA, “Determining the Need for and Content of Environmental Assessments for Gene Therapies, Vectored Vaccines, and Related Recombinant Viral or Microbial Products.” March 2015. Accessed January 15, 2024. https://www.fda.gov/media/91425/download
[36] U.S. FDA, “BLA for Minimally Manipulated, Unrelated Allogeneic Placental/Umbilical Cord Blood Intended for Hematopoietic and Immunologic Reconstitution in Patients with Disorders Affecting the Hematopoietic System.” March 2014. Accessed January 15, 2024. https://www.fda.gov/media/86387/download
[37] U.S. FDA, “IND Applications for Minimally Manipulated, Unrelated Allogeneic Placental/Umbilical Cord Blood Intended for Hematopoietic and Immunologic Reconstitution in Patients with Disorders Affecting the Hematopoietic System.” March 2014. Accessed January 15, 2024. https://www.fda.gov/media/89441/download
[38] U.S. FDA, “Preclinical Assessment of Investigational Cellular and Gene Therapy Products.” November 2013. Accessed January 15, 2024. https://www.fda.gov/media/87564/download
[39] U.S. FDA, “Preparation of IDEs and INDs for Products Intended to Repair or Replace Knee Cartilage.” December 2011. Accessed January 15, 2024. https://www.fda.gov/media/82562/download
[40] U.S. FDA, “Clinical Considerations for Therapeutic Cancer Vaccines.” October 2011. Accessed January 15, 2024. https://www.fda.gov/media/82312/download
[41] U.S. FDA, “Potency Tests for Cellular and Gene Therapy Products.” January 2011. Accessed January 15, 2024.
[42] U.S. FDA, “Cellular Therapy for Cardiac Disease.” October 2010. Accessed January 15, 2024. https://www.fda.gov/media/76587/download
[43] U.S. FDA, “Safety, Efficacy, and Pharmacokinetic Studies to Support Marketing of Immune Globulin Intravenous (Human) as Replacement Therapy for Primary Humoral Immunodeficiency.” June 2008. Accessed January 15, 2024. https://www.fda.gov/media/124333/download
[44] U.S. FDA, “Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs).” April 2008. Accessed January 15, 2024. https://www.fda.gov/media/73624/download
[45] U.S. FDA, “Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products.” August 2007. Accessed January 15, 2024. https://www.fda.gov/media/73072/download
[46] U.S. FDA, “Guidance for Human Somatic Cell Therapy and Gene Therapy.” March 1998. Accessed January 15, 2024. https://www.fda.gov/media/72402/download
[47] U.S. FDA, Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review website. Accessed January 15, 2024. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review
[48] U.S. FDA, Rare Disease Cures Accelerator website. Accessed January 15, 2024. https://www.fda.gov/drugs/regulatory-science-research-and-education/rare-disease-cures-accelerator
[49] EMA, Orphan Desigination Program website. Accessed January 15, 2024. https://www.ema.europa.eu/en/human-regulatory-overview/orphan-designation-overview
[50] EMA, Scientific Guidelines: Gene Therapy website. Accessed January 15, 2024. https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/scientific-guidelines/multidisciplinary-guidelines/multidisciplinary-gene-therapy
[51] AMED, Initiative on Rare and Undiagnosed Disease website. Accessed January 15, 2024. https://www.amed.go.jp/en/program/IRUD/
[52] AMED, Department of Regenerative Medicine and Cell and Gene Therapies website. Accessed January 15, 2024. https://www.amed.go.jp/en/program/list/13/index.html
[53] The Critical Path Institute website. Accessed January 15, 2024. https://c-path.org
[54] Orphanet website. Accessed January 15, 2024. https://www.orpha.net/consor/cgi-bin/index.php
[55] National Organization for Rare Disorders (NORD) website. Accessed January 15, 2024. https://rarediseases.org/
