Note: I used the AI Assist Drafting in Notion to generate some topics and content blocks.
Introduction
The FDA issued final guidance on the qualification process for Drug Development Tools (DDTS) in November 2020 after one round of revision and comments on the original draft published in 2014. With this document, titled “Qualification Process for Drug Development Tools [1], CBER and CDER met the mandate in the 21st Century Cures Act to issue final guidance on the process. [2] You probably think, “What are Drug Development Tools (DDTs), and why are they important?” We’ll dive into that topic in this article. A note of warning – there’s a lot of alphabet soup here, so I’ve added a glossary at the end.

DDTs Defined
The FDA defines DDTs as “methods, materials, or measures that can aid drug development and regulatory review.” [1] The five categories of DDTs are:
- Biomarkers – indicators clinicians can objectively measure and use to evaluate normal biological processes, pathogenic processes, or pharmacologic responses to therapeutic intervention. Biomarkers identify subpopulations that may benefit from a drug, assess drug safety, and monitor treatment response.
- Surrogate Endpoints – indirect measures of a drug’s therapeutic effect used instead of a direct endpoint that may take longer to measure. They are used to predict clinical benefits, reduce the duration of clinical trials, and lower development costs.
- Patient-Reported Outcomes (PROs)- measures of a patient’s health condition that come directly from the patient, without interpretation by a clinician or anyone else. They provide valuable insights into patient preferences, satisfaction, and quality of life, all relevant measures of treatment success.
- Clinical Outcome Assessments (COAs) – tools health practitioners use to evaluate how a patient feels or functions about their health condition or treatment. They are used to measure the effectiveness of drugs and can be used in clinical trials to evaluate safety and efficacy.
- Animal Models – the CURES-amended FD&C Act allows the FDA to include “any other method, material, or measure that FDA determines aids drug development and regulatory review” [1] in the DDT definition. Animal models are “animal models intended for use in the adequate and well-controlled animal efficacy studies that serve as substantial evidence of effectiveness for drugs developed under the regulations commonly referred to as the Animal Rule.” [1] These models don’t include the general ones used in drug development for proof-of-concept or safety testing.

Examples of these DDTs may be found in the “BEST (Biomarkers, EndpointS, and other Tools) Resource.” [3] A DDT is qualified in terms of its Context of Use (COU). Once qualified, the DDT “can be relied upon to have a specific interpretation and application in drug development and regulatory review,” and therefore, may be used to obtain drug approval or biologics licensure. The qualification process is entirely voluntary, and a DDT that has not been qualified or is qualified for another COU may still be used in an application where scientifically appropriate. Individual companies, biomedical research consortia, or partnerships may propose and submit a DDT for qualification.
Background
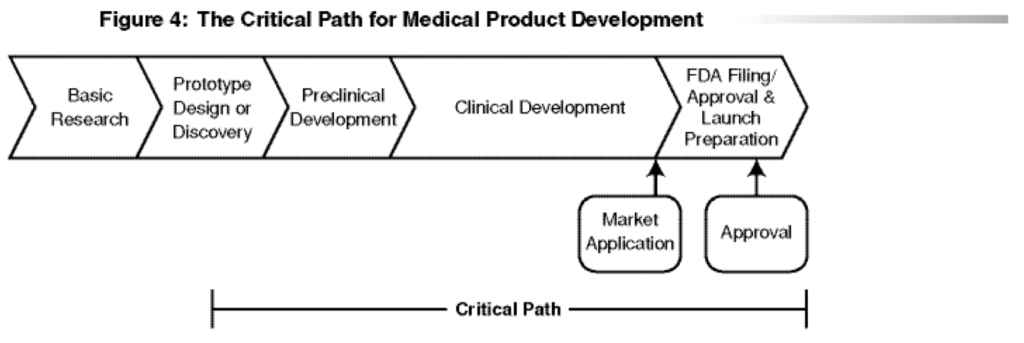
The DDT Qualification Programs (and many other programs launched over the past two decades) are an outcome of the FDA’s Critical Path Initiative (CPI). This initiative is FDA’s “national strategy for transforming the way FDA-regulated medical products are developed, evaluated, and manufactured.” [4] Launched in 2004 with the release of the report “Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products,” [5] the CPI’s goal is to improve the drug development process and reduce the time and cost of bringing new drugs to market. The report concludes, “The medical product development process is no longer able to keep pace with basic scientific innovation. Only a concerted effort to apply the new biomedical science to medical product development will succeed in modernizing the critical path.” [5] Figure 1 shows a version of the critical path from the report. DDTs fall under the categories of tools for assessing safety and demonstrating medical utility cited in the report. Progress on the CPI led to multiple FDA programs, some of which entered the FD&C Act through various laws, including CURES.

Qualification
Drug development tools are qualified by following the DDT guidance cited above. The guidance provides high-level descriptions of the three qualification programs, details on the transparency provisions in CURES that facilitate DDT development, collaboration, and use, and a list of FAQs. Additional instructions are available on the FDA’s DDT website. The first step is reviewing the existing DDTs for identical or closely-related tools. The transparency provisions make this search available via the CDER & CBER Drug Development Tool Qualification Project Search website. You can search by program, disease, therapeutic area, keyword, requestor organization, stage, and date range. Your easiest path could be joining an existing DDT project. Reviewing what is in progress and is or is not accepted will give you valuable information for your plan.
Your second step is finding out if a qualification program for your DDT exists. There are programs for three DDT types: biomarkers, COAs, and animal models. [6] Consider the newly-launched Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot program if your DDT does not fit one of these categories. [7] This program expects to accept 2-4 submissions per year. The ISTAND program personnel will also assist requestors in finding their best path forward. Another option is requesting a Critical Path Innovation Meeting (CPIM) to discuss your ideas. [8] More information on this forum, including the request form and details on the preparation package, is found on the CPIM website.
Regardless of the program, the qualification process follows a three-stage path. First, the requestor submits a LOI. Second, if the submission is accepted, the requestor submits a QP for review. QP acceptance lets the requestor proceed to the final FQP stage. Review acceptance may trigger a confidential presentation and discussion with the review committee. Requestors for submissions not accepted will receive a Determination Letter. The Qualification Process guidance includes a complete description of the process. [1] All DDT requests must be electronic via the FDA’s NextGen Collaboration Portal. [9] Post-qualification submissions follow the same three-stage process.

International Support
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) supports developing and using DDTs in drug development programs, including biomarkers, surrogate endpoints, and clinical outcome assessments. The ICH has published several guidelines on the use of these tools in drug development, including the ICH E6(R3) Good Clinical Practice draft guideline, which includes PROs and COAs as examples of Data Acquisition Tools (DATs) to collect clinical trial data on the safety and efficacy of drugs. [10] The ICH Reflection paper “Proposed ICH Guideline Work to Advance Patient Focused Drug Development.” also cites COAs as targets for new ICH guidance. [11] We can expect increased international support for DDTs as regulators see applications with data from qualified DDTs.
Conclusion
The FDA process to qualify drug development tools accelerates the development of safe and effective drugs for patients. These tools, including biomarkers, surrogate endpoints, patient-reported outcomes, clinical outcome assessments, and animal models, help identify subpopulations that may benefit from a drug, assess drug safety, and predict clinical benefit. These tools should revolutionize the drug development process by making it faster, more efficient, and more cost-effective. Companies developing new medicines would be wise to track and use the DDTs relevant to their therapeutic area.
NextGen Collaboration Portal
The FDA‘s NextGen Collaboration portal is a valuable tool for those in the regulated industry who need to communicate with the FDA quickly and securely. The system allows for real-time submission, review, and response to communications such as pre-assigned number requests, controlled correspondence, and drug supply notifications and is constantly adding new functions.
https://krogersconsulting.com/medical-products/cderdirect-nextgen-collaboration-portal/
References
[1] USFDA, “Qualification Process for Drug Development Tools: Guidance for Industry and FDA Staff.” November, 2020, accessed May 1, 2023. https://www.fda.gov/media/133511/download
[2] Public Law 114–255, “21st Century Cures Act,” December 13, 2016, Section 3011. Accessed May 26, 2023. https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.pdf
[3] FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring (MD): Food and Drug Administration (US); 2016-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK326791/. Co-published by National Institutes of Health (US), Bethesda (MD).
[4] USFDA Critical Path Initiative website, accessed May 1, 2023. https://www.fda.gov/science-research/science-and-research-special-topics/critical-path-initiative
[5] USFDA, “Innovation/Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products.” March, 2004, accessed May 1, 2023. http://wayback.archive-it.org/7993/20180125035500/https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/UCM113411.pdf
[6] USFDA Drug Development Tools website, accessed May 1, 2023. https://www.fda.gov/drugs/development-approval-process-drugs/drug-development-tools-ddts
[7] USFDA Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program website, accessed May 26, 2023. https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/innovative-science-and-technology-approaches-new-drugs-istand-pilot-program
[8] USFDA Critical Path Innovation Meeting website, accessed May 1, 2023. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/critical-path-innovation-meetings-cpim
[9] USFDA Drug Development Tool Submissions Process website, accessed May 26, 2023. https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/drug-development-tool-submissions-process
[10] ICH E6 R3 EWG, “Good Clinical Practice (GCP),” Draft Guideline, released 19MAY2023, accessed May 26, 2023. https://database.ich.org/sites/default/files/ICH_E6%28R3%29_DraftGuideline_2023_0519.pdf
[11] ICH Reflection Paper, “Proposed ICH Guideline Work to Advance Patient Focused Drug Development,” Endorsed on 02JUN2021, Accessed May 26, 2023. https://admin.ich.org/sites/default/files/2021-06/ICH_ReflectionPaper_PFDD_FinalRevisedPostConsultation_2021_0602.pdf
Glossary
BEST – Biomarkers, EndpointS, and other Tools
CBER – Center for Biologics Evaluation and Research
CDER – Center for Drug Evaluation and Research
COA – Clinical Outcome Assessments
CPIM – Critical Path Innovation Meeting
CURES – the 21st Century Cures Act of 2016
DATs – Data Acquisition Tools
DDTs – Drug Development Tools
FD&C Act – Food, Drug, & Cosmetic Act, as amended.
LOI – Letter of Intent
QP – Qualification Package
FQP – Full Qualification Package
PRO – Patient-Reported Outcomes
