Katrina’s Blog™
Insightful News and Commentary
On leadership, product innovation, talent cultivation, and ecosystem development across drugs, devices, diagnostics, and digital health tools.
Join us as we explore the latest trends and share expert insights to help you navigate the ever-evolving landscape of healthcare innovation.
Let’s embark on this journey of transformation together!
What is the Division of Applied Regulatory Science?
Oct 23, 2018 | Uncategorized
and what do they do?Part of the Office of Clinical Pharmacology, the Division of Applied Regulatory Science (DARS) performs applied research to “develop and evaluate novel tools, standards, and approaches to assess the safety, efficacy, quality, and performance of...
Question-based Review Process Improves ANDA Submission and Review
Oct 21, 2018 | Uncategorized
Following the release of the report “Final Report on Pharmaceutical cGMPs for the 21st Century – A Risk-Based Approach” in 2003, the Office of Generic Drugs (OGD) recognized that its process for review of ANDAs needed an update to meet the new risk-based approach and...
Webinar on Quality Systems Basics for Device Manufacture
Oct 18, 2018 | Uncategorized
The Division of Industry and Consumer Education (DICE) in the Center for Devices and Radiological Health (CDRH) will be hosting a workshop for medical device manufacturers on November 6, 2018. The workshop, which has a webinar format, will focus on 2 parts of the...
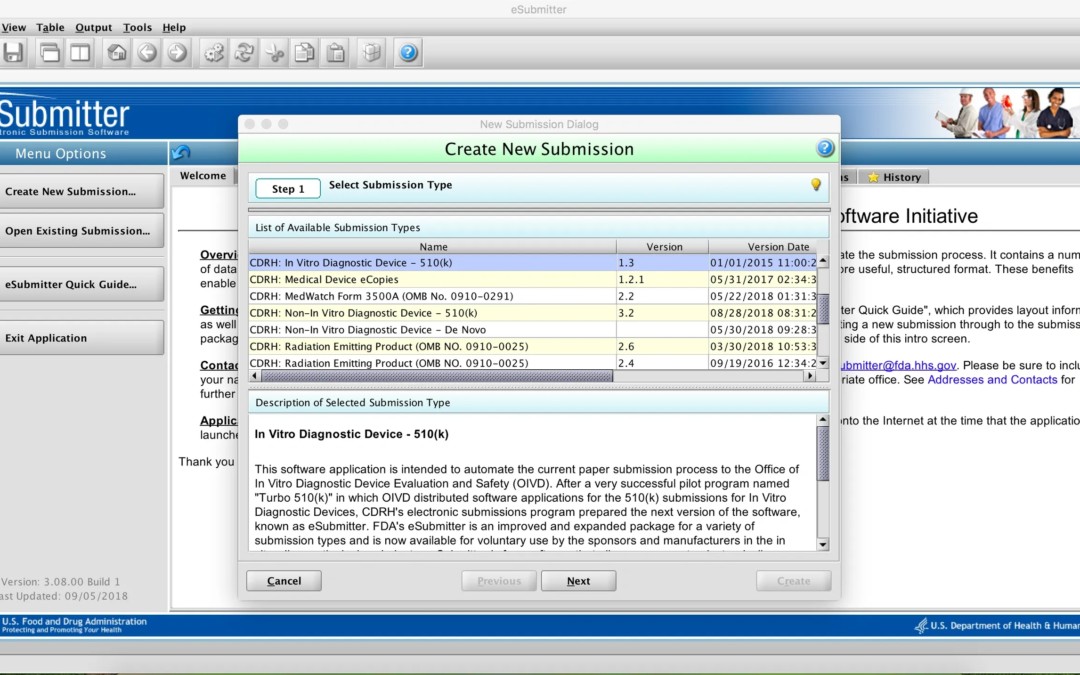
What is the Quality in 501(k) (“QUIK”) Review Program Pilot?
Oct 16, 2018 | Uncategorized
CDRH is currently running a pilot program to accept 510(k) premarket notification submissions packaged using the eSubmitter tool (see my previous post). The purpose of the pilot is to collect information to assess the value of the template in facilitating review and...
Collaborative Communities Initiative for Medical Devices
Oct 14, 2018 | Uncategorized
One of the current strategic priorities for CDRH is the creation of Collaborative Communities to allow interested stakeholders (both private and public, including the FDA) to work together medical device challenges. These communities may be convened by any...
Categories
Latest Posts
Today is Rare Disease Day
Feb 28, 2019 | Uncategorized
The last day of February is designated Rare Disease Day, a campaign to raise awareness of rare diseases and their impact on patients, caregivers, and society. On this day local organizations host events to improve public knowledge of rare diseases and advocate for...
What Are All These Device Review and Approval Pathways? Part 6
Feb 26, 2019 | Uncategorized
Premarket ApprovalMost devices categorized as Class III (devices that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury) are required to go...
What Are All These Device Review and Approval Pathways? Part 5
Feb 24, 2019 | Uncategorized
Strengthening the 3rd Party 510(k) Review ProgramThe 3rd Party (3P) Review Program is an alternative process for manufacturers of medical devices to speed decisions on premarket applications for low- to moderate-risk and less complex medical devices. Under the program...
Draft Guidance on Rare Disease Drug Development
Feb 21, 2019 | Uncategorized
The CDER/CBER divisions of the FDA released a draft guidance in February 2019 describing various approaches to addressing challenges common in rare disease drug development. This latest guidance focuses on issues faced by sponsors in clinical trials; issues in early...
