Katrina’s Blog™
Insightful News and Commentary
On leadership, product innovation, talent cultivation, and ecosystem development across drugs, devices, diagnostics, and digital health tools.
Join us as we explore the latest trends and share expert insights to help you navigate the ever-evolving landscape of healthcare innovation.
Let’s embark on this journey of transformation together!
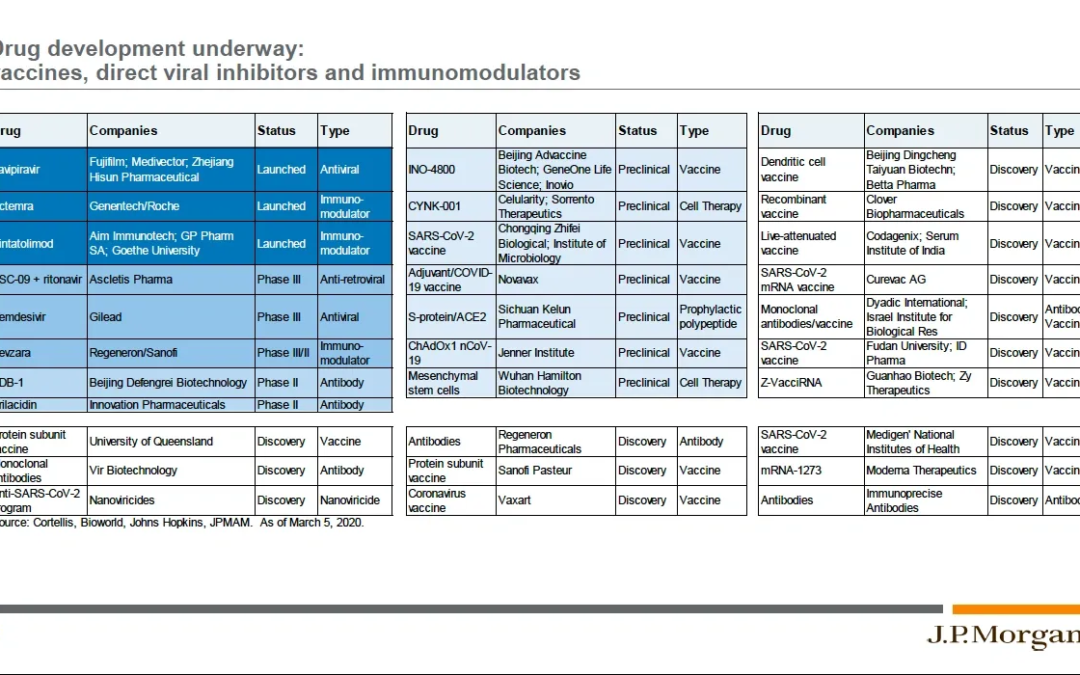
Outbreak! Combating the 2019-Novel Coronavirus (COVID-19)
Mar 24, 2020 | COVID
Chapter 6 – Biomedical Mobilization Against COVID-19 The response of our biomedical community to the novel coronavirus outbreak has been impressive, and is illustrated in this slide from a JP Morgan webcast on COVID-19 and the Markets held on March 20th...

Standards Organizations Play a Role in Medical Product Regulation
Mar 19, 2020 | Medical Products, Medicine, Product Development
Regulatory bodies worldwide make use of consensus standards developed by independent organizations to establish best practices and conceptual harmony on specialized areas of expertise. Standards organizations for drugs include the regional Pharmacopeia USP, EP, and JP...

What are the Pharmacopoeia?
Mar 17, 2020 | Medical Products
Traditionally, pharmacopoeia is a book of medicinal drugs with their effects and directions for use. Today, the pharmacopoeia are regional organizations devoted to creating and maintaining standards to ensure public health. Part of that mission includes maintaining a...

Using Inspection Procedures & Guides as a Preparation Tool for Medical Device Inspection
Mar 12, 2020 | Product Development
If you are involved in the development or manufacturing of a medical product, sooner or later, you will face a regulatory inspection. The individual regulatory agencies publish many of their inspection procedures online, and these documents are valuable references for...
Three-Tiered Approach to Regulation of Next Generation Sequencing
Mar 10, 2020 | Product Development
With the recent approval of three new next generation sequencing diagnostic tests for tumor profiling, CDRH also announced their approach to regulating such tests. There are three levels of validated biomarkers addressed in each of the market authorizations for the...
Categories
Latest Posts
Katrina’s Summer 2021 Reading List
Jun 7, 2021 | Business, Leadership
Those who follow my feeds will have guessed by now that reading is my superpower (when I haven't shared that strength in person). One of my favorite summer activities was heading down to the public library for a stack of books, and I'll shout out to the Jervis...
Special Post: Life Sciences in the Northwest
Mar 30, 2021 | Uncategorized
This week we’ll be attending Life Science Innovation Northwest (LSINW), a virtual two-day meeting of investors, public and private life science organizations, research institutions, scientists, entrepreneurs devoted to the life science ecosystem in the upper left...
Katrina’s Leadership Reading List for 2021
Dec 29, 2020 | Uncategorized
2020 has been a tough year in many ways, but we’ve also seen multiple opportunities for constructive change. Working from home and other flexible arrangements are here to stay. Widespread compliance with stay-at-home orders has shown us we can change atmospheric...
Strategic Planning for Medical Products
Dec 1, 2020 | Product Development
Part 1 - US Pharmaceutical Regulatory PathPharmaceuticals and medical devices are regulated in the USA by the FDA. The regulations started to diverge in 1976 following the passage of the Medical Device Amendments to the Food, Drug, and Cosmetic Act. Each type of...
